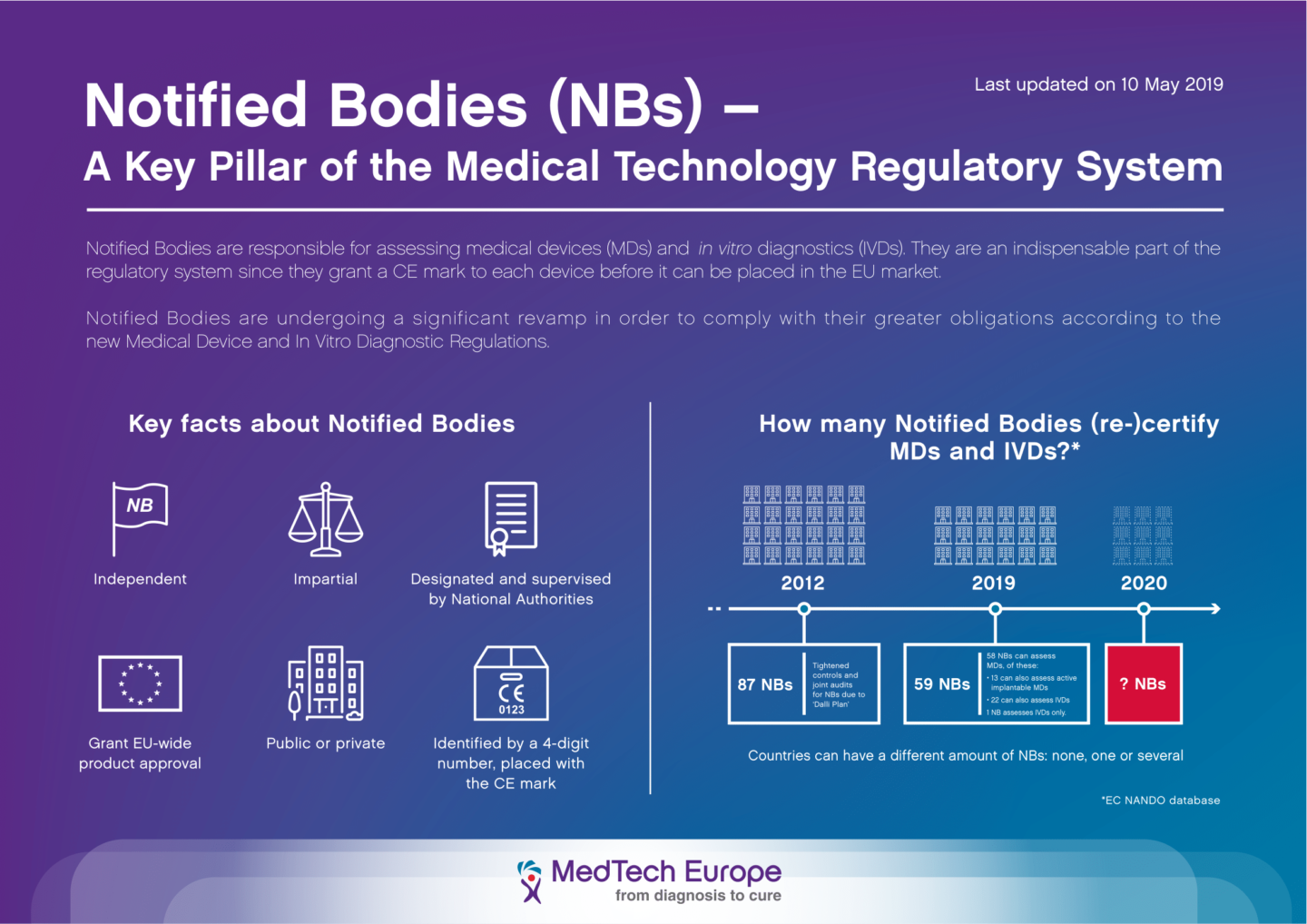
What Does the CE Mark Mean, and What is its Purpose? - Medical Device Academy Medical Device Academy

New MDCG guidance on temporary extraordinary measures related to medical device Notified Body audits during COVID-19 quarantine orders and travel restrictions | medicaldeviceslegal
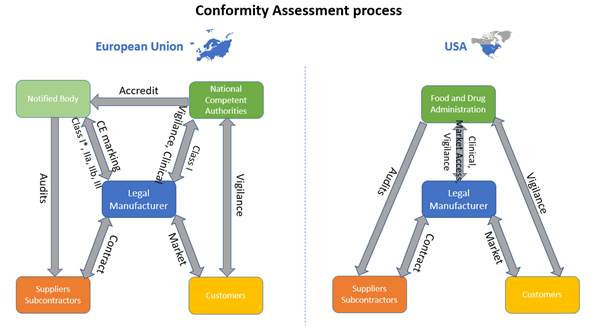
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito







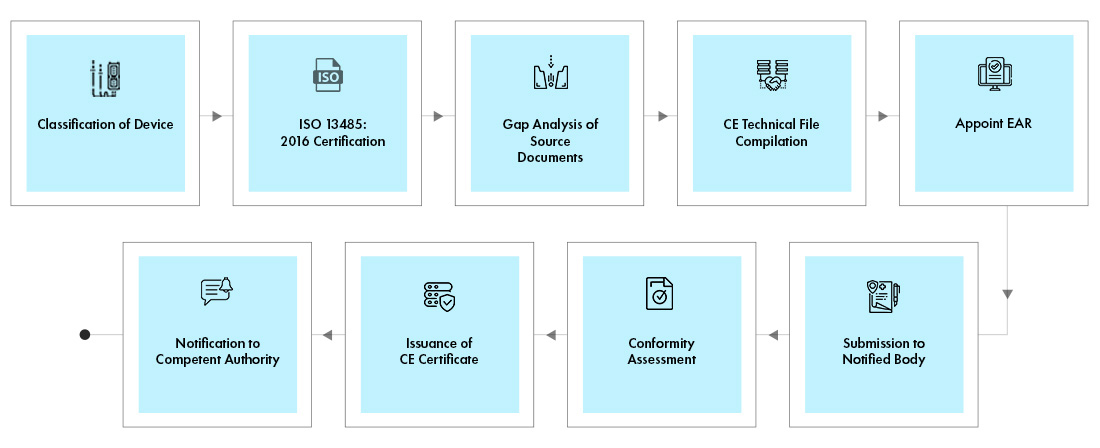
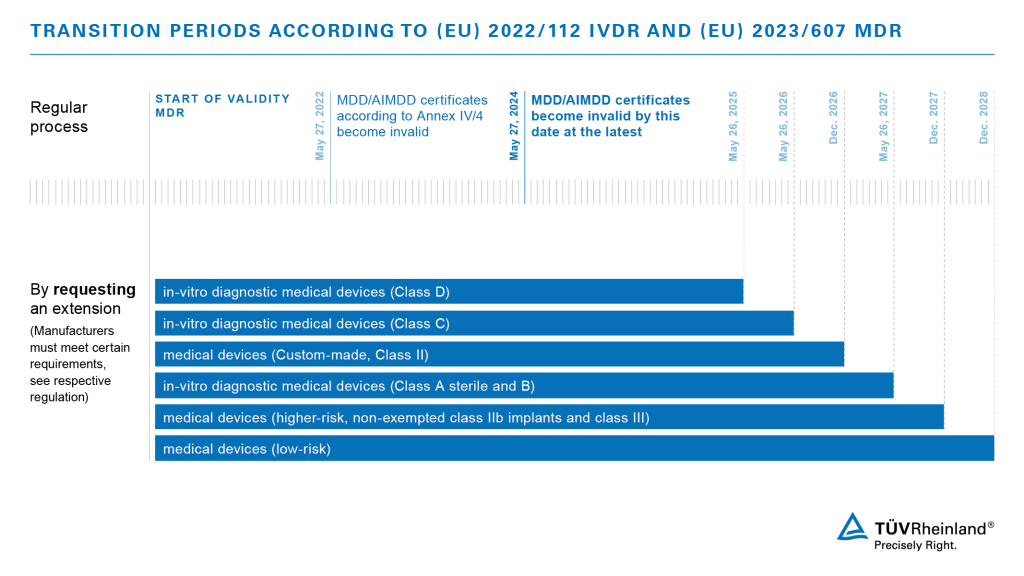



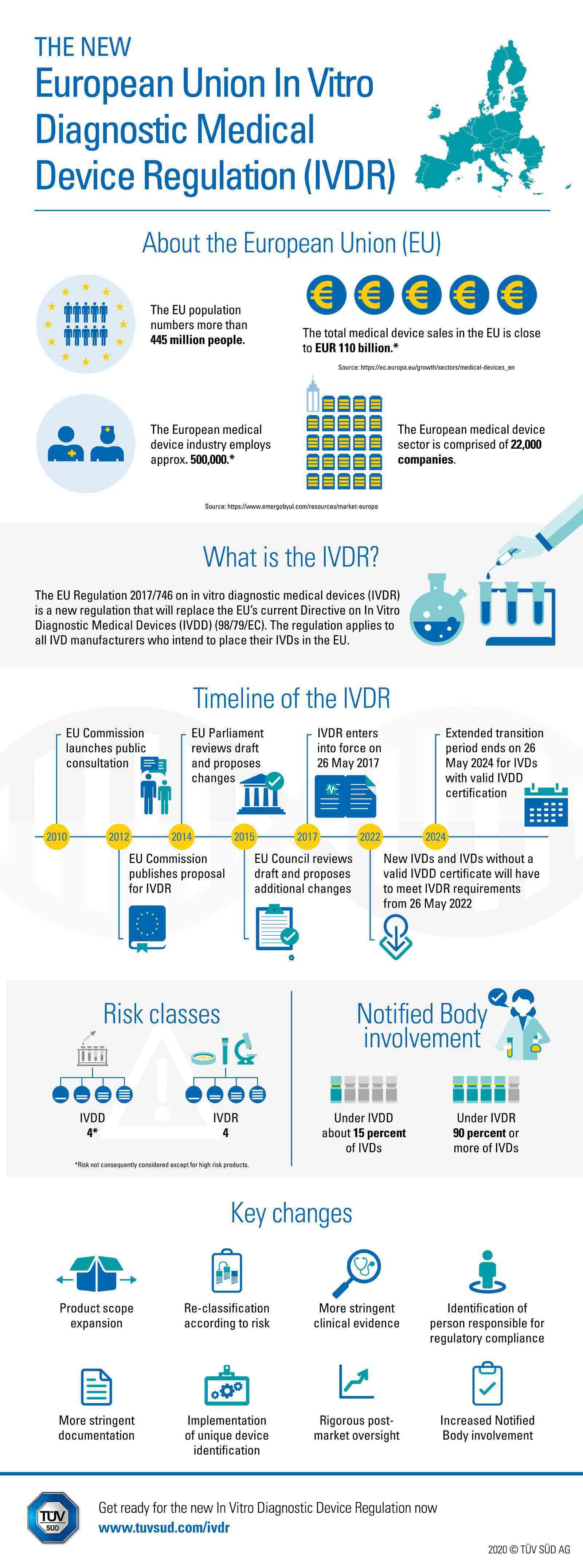
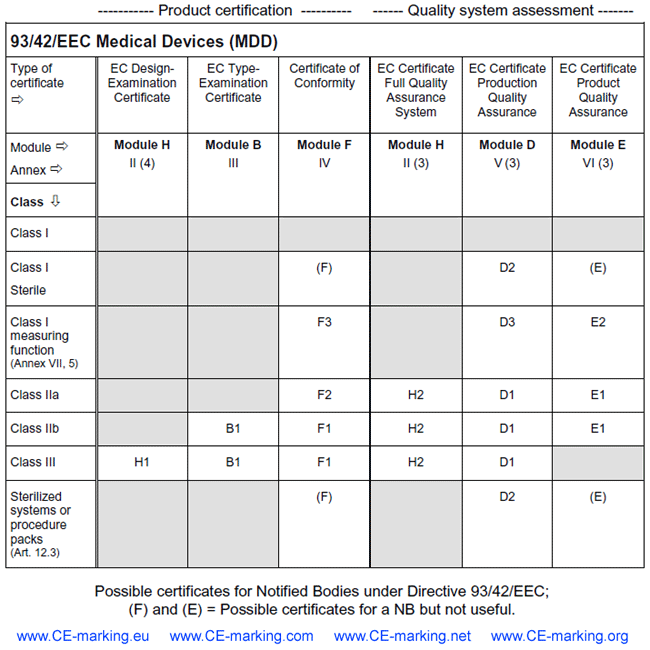
/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)