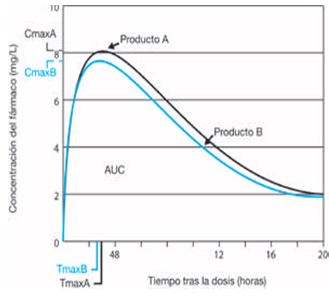
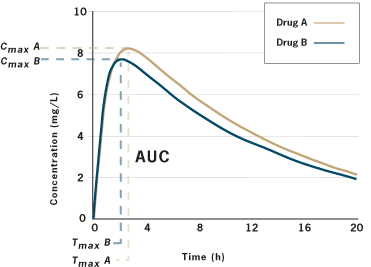
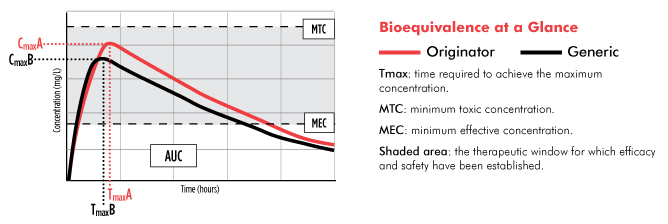
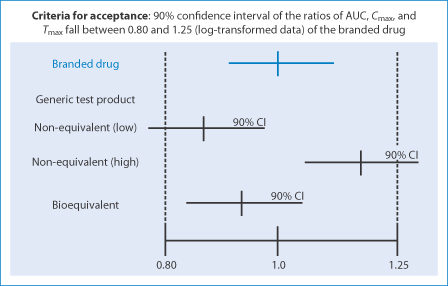
Comparing Generic and Innovator Drugs: A Review of 12 Years of Bioequivalence Data from the United States Food and Drug Administ
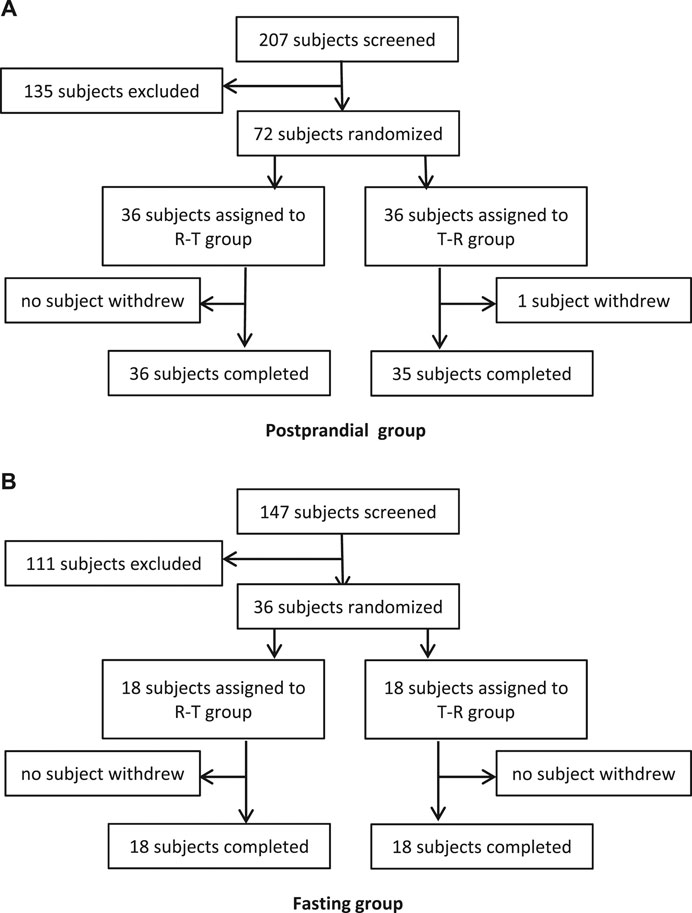
Frontiers | Pharmacokinetics and Bioequivalence of Rasagiline Tablets in Chinese Healthy Subjects Under Fasting and Fed Conditions: An Open, Randomized, Single-Dose, Double-Cycle, Two-Sequence, Crossover Trial

Assessment of the impact of partial area under the curve in a bioavailability/bioequivalence study on generic prolonged-release formulations - ScienceDirect
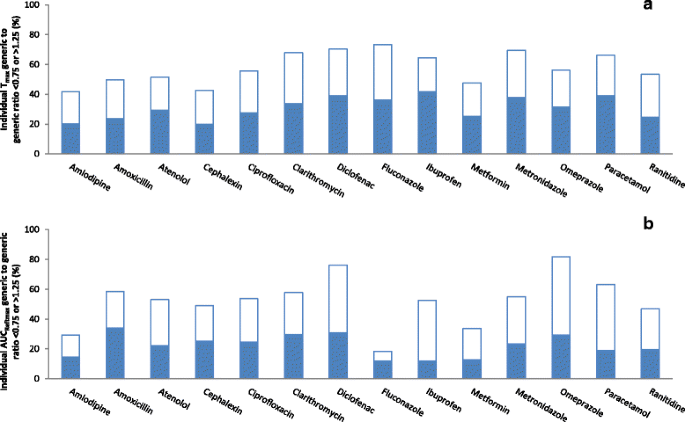
Generic-reference and generic-generic bioequivalence of forty-two, randomly-selected, on-market generic products of fourteen immediate-release oral drugs | BMC Pharmacology and Toxicology | Full Text
![PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/25e18dc07f478ecfade16b741d6768a6cb3da9b3/3-Table1-1.png)
PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar